Chelation – a new form of blood letting?
Linking disease, chelation and arsenic - The Arsenic Files Part 7
If we were accidentally exposed to a toxic metal, we would expect our doctor to prescribe a drug to get it out of our body. It seems though that whilst we are aware arsenic is consistently showing high in hair mineral tests, there is little to no awareness (or acknowledgment) of this in healthcare sectors globally. Do they really not know, or is it a ‘need to know’ sort of topic? Are doctors directed one way or another according to symptoms in an attempt to mitigate effects? In this article we look at how chelating agents, or heavy metal/metalloid removers, work, what the problems are in administering them and how diseases seem to improve when they are administered.
Chelators work by attaching to metal ions using one or more electron donor atoms. Sulphur, nitrogen and oxygen are the main donor atoms for chelation. The success of a chelator depends on the ‘net donor charge’ in the complex. If the charges between the two parts only form a weak bond, the chelate (target metal) can be shifted out of one area of the body and released into another, even in to the brain. Heavy metal chelators often form a multidentate ring, a ring with many teeth or atom attachments. The ring and donor atoms form a tight hold on the chelate so it can be removed. Other chelators have one or more ligands (joins which form through exchange of electrons) which join to their target ions. Heavy metals may require more than one chelating molecule to bind them adequately.
As these agents work using ion and electron exchange they can also sometimes remove essential metals as well as toxic heavy metals and metalloids. Ideally drugs must be designed to strip out the target ion rather than anything essential, chelation drugs must not be toxic in themselves, should be easily dissolved in lipids and, if not administered intravenously, should be easily absorbed in to the blood stream from the gut.
EDTA
One of the better known chelating agents is ethylenediaminetetraacetic (EDTA). It is used in many applications including preservative for cosmetics, blood and kidney function analysis, in root canal surgery, ion exchange chromatography to separate lanthanide metals (atomic numbers 57-71) and of course metal removal.
To remove metals or minerals, EDTA holds a single ion in place using two nitrogen atoms and four oxygen atoms in a six sided complex, known as a hexadentate ligand. Ligand comes from the Latin word ‘ligandus’, which means ‘to bind’ (etymonline.com). Figure 1 what the complex looks like before it attaches to an ion, and afterwards. You can see how the molecule closes around the metal ion.
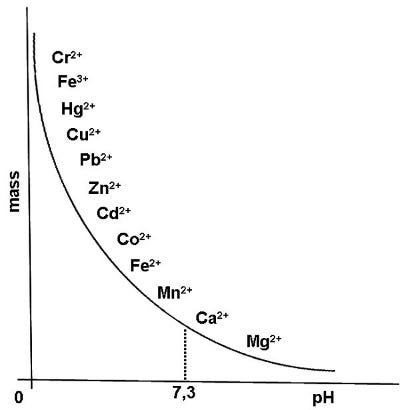
As we noted above, EDTA can bind both to toxic and essential metals in the body. This chart (Figure 2) shows some of the metals. To the left top of the chart are the metals which EDTA is more attracted to and to the right lower part are the metals it is less attracted to. EDTA is usually administered in to the vein (intravenously), which allows it to be excreted through the kidneys without going through the liver. As we can see, the more acidic the environment, the easier the removal of the more toxic metals, but as the pH starts to move towards alkalinity, essential metals are also targeted. Zinc seems particularly vulnerable to chelation and must be replaced during therapy.
Side effects of EDTA administration are headache, nausea, vomiting, fever, aches and cramping muscles, low blood sugar, low blood pressure. It can also chelate calcium, causing a drop in levels which can lead to heart arrythmias and brain related effects including seizures, a result of abnormal electrolyte levels. EDTA can also suppress blood cell production causing anemia and white blood cells, whilst increasing band cells from bone marrow causing temporary inflammation. So the benefits of using EDTA must be measured carefully against patient health and the potential drawbacks.
Iron chelation
In the chart above EDTA shows different affinity with two forms of Iron (Fe). Fe3+ is more positively charged, and therefore more strongly attracted to the nitrogen atoms in the EDTA complex than Fe2+. When arsine gas enters the blood through the lungs it causes damage in oxygenated haemoglobin rather than non oxygenated. This is because its hydrogen atoms are attracted to the oxygen atoms in the haemoglobin complex.
Once arsine gets in to the haemoglobin complex it can cause the release of ferriprotoporphyrin IX, which is the hexadentate structure (six toothed or porphyrin structure) which holds iron in its Fe3+ form. This iron binding compound is then floating around in the blood stream unable to do its job of transporting oxygen to cells. Not only does this mean less available oxygen, but it also creates a log jam of excess iron containing molecules all trying to get in to the liver to be processed. The liver, heart and other organs can build up high levels of free Fe3+ iron, which can be toxic if levels stay high for long. EDTA can be used to soak up this excess iron, taking the stress off the liver particularly, and allowing it to reprocess the remaining iron and pass it through to the bone marrow for more blood cells to be produced. EDTA targets Fe3+ iron before it does Fe2+, so it is targeted to a degree towards the iron form that is released by arsenic.
A 2007 Canadian study estimated that 1 in 227 caucasian people had a genetically triggered, excessive build up of iron (hereditary hemochromatosis). They noted that most of the people with the condition were male. Many patients were alcoholics, chronic ‘viral’ hepatitis, diabetes, elevated liver enzymes, haemoglobin and serum ferritin (blood iron) and carried the single genetic mutation C282Y. However, we see a similar effect in Covid-19 and in arsine exposure (The Battle Field), an overload in cells and tissues, release of free toxic iron carrying heme in to the blood, heme induced liver damage and other related conditions.
There seems to be a significant amount of research surrounding the chelation of iron, and resulting drugs including deferoxamine, deferiprone, and deferasirox. These are used also to treat the condition Thallasemia, which is a different type of iron overload usually found in Southeast Asia, the Middle East, and the Mediterranean due to the mutation of the H63D gene. The difference in gene mutation is apparently due to the anthropological differences between races.
In our enquiries we noticed a marked increase in people reporting blood conditions in our relatively small contact group over the last 10 years. In a UK study between 1987 and 2002 the UK General Practice Research Database saw a two fold increase in patients diagnosed with hemochromatosis. The study recognised that the condition is on the increase but gave no suspected cause. The steady increase suggests the genetic change may be a result of exposure to arsenic or other toxins, rather than genetic mutation being the root cause of the condition. As clinicians do not, as a matter of course, test for arsenic toxicity at the same time as doing blood analysis it is unlikely that the two have ever been connected. If analysis were done concurrently, a connection may well become apparent.
Iron chelation therapy has been shown to be effective in Parkinson’s disease, Alzheimers, hepatitis, cardiovascular disease and seemingly a long list of other conditions.
Clotting
Because of EDTA’s affinity with calcium, and calcium being a main proponent in clot and plaque formation, it has been deemed successful in studies treating cardiovascular disease. Patients experienced improvements in a number of cardiovascular conditions, and improvements in lung function (though may not be effective for all lung conditions).
Calcium works with vitamin K and fibrinogen to form clots in injured blood vessels and around dead cells. On rare occasions red blood cell death has happened in the presence of EDTA (thrombocytopenia), which can make clotting more likely . If EDTA adsorbs free calcium but clots are still taking place, it might indicate much higher levels of free calcium present in the blood stream than usual, potentially from arsine induced red blood cell death.
In a study of arsenic effects on calcium, it was found that calcium levels inside the cells decreased significantly on exposure to arsenic. This disturbance in calcium levels inside cells can also lead to cell death, which of course would release other ions in to the blood stream further triggering the clotting process. If a person’s blood has been exposed to arsenic before the administration of EDTA, it could well be that excess calcium in the blood stream continues to promote blood clotting in spite of the presence of EDTA, rather than because of.
Of course, we must not forget that EDTA also chelates arsenic. It might be the ability of the drug to remove the cause – arsenic – and the products of its works – free iron and other excess free ions including calcium, that allows it to be such a successful all round chelator.
British Anti-Lewisite
Dimercaprol, or British Anti-lewisite, was formulated during WWII as an antidote to Lewisite, a gas combining chlorine and arsenic, designed as a chemical weapon. Lewisite has three chlorine molecules, two of which are attached to an arsenic molecule. Chlorine relaxes cell walls and allows arsenic to enter. It was primarily a blistering agent, of the skin and mucus membranes of the respiratory tract.
Dimercaprol is administered through the muscle, as close to the time of exposure as possible or within 24 hours. It is currently used to chelate gold, mercury, arsenic, antimony, bismuth and thallium. It is not used to chelate cadmium, iron or selenium as these compounds can build up in kidney tissue, possibly because the bond between them and the chelating agent is not strong enough.
Dimercaprol has two sulphur atoms (sulphydryl groups), which bind to the arsenic atom as seen in this image The complex is then excreted through the liver (bile) and kidneys (urine) . The list of side effects include: severe headache, drowsiness, nausea, vomiting, pain/pressure throat and chest, anxiety, rapid heartbeat, high blood pressure, burning sensations (mouth, throat, penis), eye irritation, runny nose/watery mouth, urination issues (kidneys). Abcesses that are sterile can also appear at the injection site and the compound can cause allergic type reactions. It can also mobilise and move lead to the brain.
Interestingly Dimercaprol is used for the treatment of Wilson’s disease, which is in part characterised by the increase in copper levels, apparently due to a ‘rare liver disease’ in patients. The treatment helps to a certain degree but the effects soon wear off, requiring repeated administration. This means that a mechanism in the body causing copper build up could be alleviated but not stopped entirely. Approximately 10-15% of patients also suffered from haemolytic anemia (red blood cell death) apparently due to copper toxicity. Of course where we see an overload in some patients we can also see an overload of iron, which can be with or without high levels of red blood cell death. The rise in free iron levels, followed by haemolytic anemia are seen in arsenic poisoning and radiation poisoning.
In arsenic poisoning the liver can struggle significantly due to the metabolism of arsenic, which could lead to a build up of other molecules competing for functioning hepatocytes to metabolise them (liver cells). The fact that we can also see copper increases and iron increases and decreases in Wilson’s patients suggests that arsenic could also be considered as an inducer of the disease.
Dithiols – double sulphur groups as chelators
Double sulphur compounds (dithiols) have good ability to chelate arsenic, copper, chromium and zinc. Single sulphur atoms are much less effective. Tap on the drug name to see the molecule formations for Succimer (DMSA), Dimercaprol (BAL) and Unithiol (DMPS). They all have slightly different arrangements and can also chelate mercury and lead, though Dimercaprol is not and effective mercury chelator. Succimer does not attach to iron so would be a good choice in a patient with normal to low iron levels.
Both Succimer and Unithiol use a ligand type of attachment to link to its target metal/metalloid. This attachment can be seen in figure A here. Side effects of this treatment include nausea, vomiting, diarrhoea (similar to a toxicity reaction), rashes, and sometimes high fever.
You may have read the article ‘From the Gut with Love’ where we talk about arsenic being specifically attracted to sulphur. Dithiols can bond more tightly with arsenic. Similar dithiol groups are seen in alpha lipoic acid containing foods. Again, if acutely exposed to high levels of toxic metals, the recommendation is to start chelation therapy as soon as possible after exposure, from minutes to hours.
Because we suspect long periods of low level lung absorption of arsine gas, it would not be practical for repeated use of this form of chelation unless there were some way to space treatment to maximum effect. Alpha lipoic acid has a natural dithiol, and has been tested in experiments opposite British anti-lewisite for effectiveness in detoxification of arsenic species. B12 has a porphyrine (structure that holds heavy metal securely) that is also known to enable removal of heavy metals including iron. Both B12 (Methycobalamin) and Alpha lipoic acid are natural chelators which we can derive from food on a meal by meal basis. In the face of arsine gas, this type of daily support is probably quite important.
We know from our study on Probiotics that bacteria have the ability to metabolise mercury, arsenic, other toxic metals and of course essential metals including iron. If any is present in high quantities in these bacteria it is likely they will be used in the structure rather than the essential element cobalt. Therefore ensuring we get more B12 through diet may compensate for the loss from chelation by B12 in the gut. In fact it has been noted that people with a high heavy metal load are sometimes low in B12.
Alzheimers, Clioquinol and Copper
Alzheimers patients have been shown to be high in mercury. Cadmium toxicity has also been shown to alter short term spatial memory (in mice) and of course arsenic has been hypothesises as being linked to key markers for dementia and alzheimers. In treatment of alzheimers a number of chelation drugs have been trialed with the most successful being from a group of 8-hydroxyquinoline analogues, one of which is Clioquinol.
Clioquinol, the drug used to treat protozoa/parasitic infection in Japan, was blamed for low copper levels and SMON (neuropathy) alongside low/high iron levels, is one of this class of drugs. Seb Powell talks about SMON more here. Now rediscovered as a chelator, it appears Clioquinol has moderate chelating effects on iron, zinc and copper but can act as an ‘ionophore’ (similar to the porphoryn that carries metal ions around the body) moving zinc and copper to inside cells instead of removing them. The improvements in Alzeheimers patients seems to be down to the repositioning of zinc and copper restoring some synaptic function. However, other studies found the drug led to temporary cognitive impairment.
The ‘green’ effects caused by the drug in SMON as detailed by Seb, may well have been down to an increase in iron chelate or even a repositioning of copper.
We are not in the least bit surprised that copper also binds to cysteine (sulphur containing) proteins in the same ways as zinc. Meaning that copper can also be displaced by arsenic. Copper is an integral part of the production of energy in cells. It is the vehicle that allows the transfer of electrons. Copper also aids in the production of melanine which helps us tan, helps create connective tissues and of course helps transport iron. It also, importantly in relation to Alzheimers, keeps our brains and nervous system healthy! Symptoms of multiple cclerosis have been triggered in mice with the administration of Cuprizon, a copper chelator, as Seb discusses here. The medical and nutritional implications of this we will look at in more detail in a follow up article to ‘From the Gut with Love’.
As we have seen above, chelating agents seem to have a remarkable effect on a wide range of seemingly unrelated diseases, yet they all seem to have one factor in common. An excess or a deficit of certain metals and minerals in the body and the presence of some metals in areas they should not be, indicating they have been released in to the blood stream in large quantities at some point or over time. An excess of which can be remedied with nitrogen, oxygen and sulphur based chelating drugs.
The body is a clever organic machine. It self regulates, and is able to ensure the right amount nutrients are circulating to perform all the essential functions. The introduction of toxins create an imbalance in the body by way of cell destruction and metal/mineral appropriation as we have seen from our work on arsenic. Genetic mutations must have a trigger, as we have seen in bacteria. Only the presence of arsenic and other toxic heavy metals, is there a need for horizontal gene transfer, and operon activation to ensure the bacteria survives.
Before you run out to sign up to chelation therapy, read the next articles on antibiotics, asthma inhalers and other drug classes. They have more uses than ‘killing bacteria’ and resolving ‘asthma’ - whatever asthma actually is! We will also take a look at some drugs which are designed to reduce production of metal containing proteins in our bodies. Rather than removing metals some can also prevent their uptake.
Thanks to Seb as always for your contributions to this series.




Hi caroline, there is a popular supplement for heavy metal detox called Clean slate from Roots and ingredients say Silicon dioxide with vit c- from your earlier work, i deduced this may actually attract more arsenic in the body. Do you agree? Also you do not mention zeolites here, which are popular for chelating heavy metals. What do you think of zeolites? Thank you
I did understand that Vitamin B12 is important, I'm confused on the chelation therapy, seems like a good thing but the too much iron thing it causes could out weigh the good. Am I on the right track?