Surveying the Land
Before we dive in, it is important to emphasise that arsenic has a half life of around 3-6 hours in the blood and the metabolites (what arsenic is broken down in to by the liver) have half lives of 2-4 days. That means, from the point of exposure, to reducing the total amount by half in the blood is 3-6 hours and so on. Every 3-6 hours after that, the remaining amount, that hasn’t got stuck somewhere or transformed in to something else, will be reduced by another half.
Arsenic doesn’t just fly through the body and in to the kidneys, it must go through processes to get there first and this is where other arsenic species may get distracted and be attracted to specific targets in the body. There are two main routes of entry, the gut and the lungs. As arsenic is odourless and colourless it is difficult to tell if and when we are inhaling or ingesting it. Therefore understanding the routes of entry and how the body processes it, goes some way to recognising a level of toxicity.
The arsine gas molecule enters into the lungs, through the heart, around the body in the cardiovascular system and through the kidneys, liver and spleen. Heavy metals are primarily excreted through the kidneys. The arsenic-based intruder, whatever form it might take at the time of excretion, can be detected in the urine from 3 hours to around day 4 from point of exposure. The ideal level of arsenic in the urine is between 0 to 19 ug (micrograms per litre) or 0.19 ppb. Urine arsenic levels are most accurately measured within 24 hours of suspected exposure.
There is some talk of a little bit of arsenic being an essential nutrient in the body, however there is no real evidence for this claim. The only exception being arsenic trioxide for treating acute promyelocytic leukemia and even then the short term positives may be outweighed by long term negatives.
A magnifiying glass doesn’t quite cut it
Arsenic comes in a solid and gas. Solid arsenic pentoxide (V) and trioxide (III) can be slightly dissolved in water. Arsine gas can be dissolved into water (or rain) and can be stored and transported in canisters of liquified compressed gas. The gas molecule, AsH3, makes a three sided pyramid, the arsenic atom at the top and three hydrogen atoms as the legs.
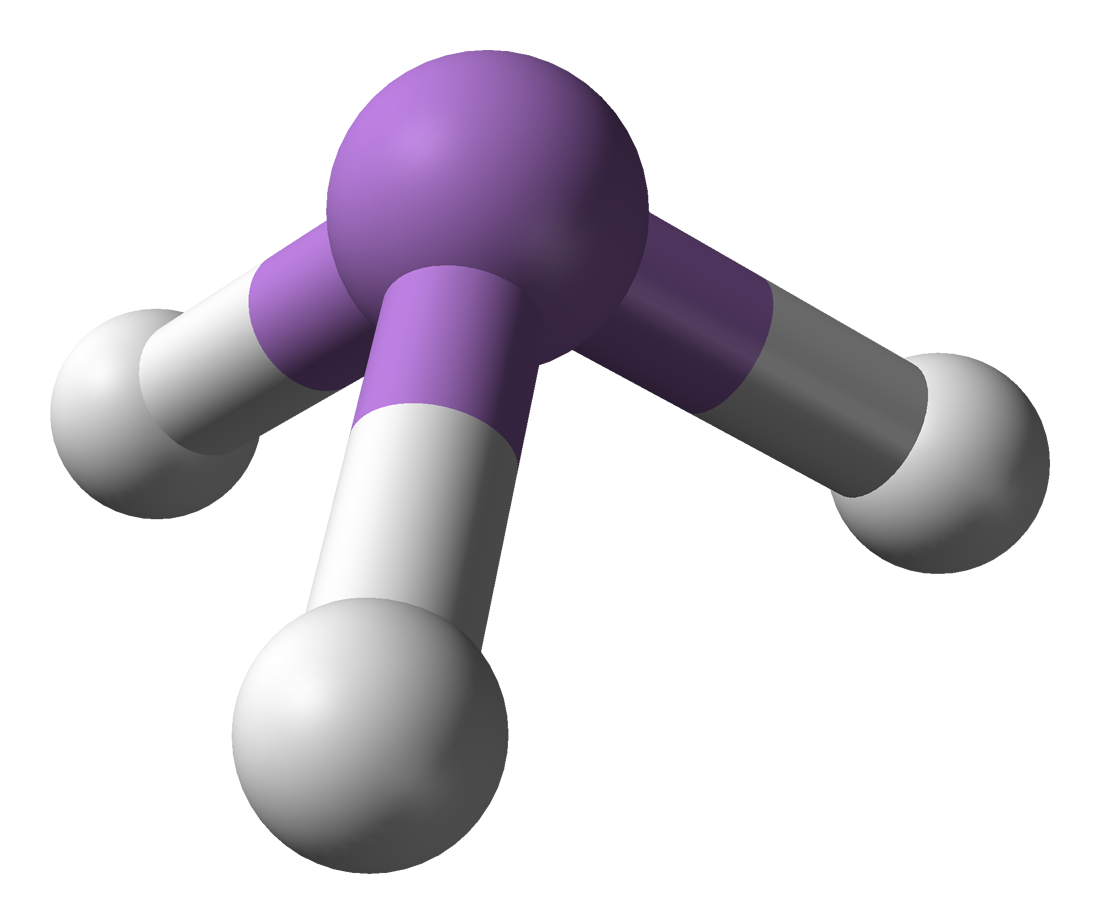
Though arsenic trioxide is soluble in water and can create an inhalable dust, it is a relative heavy weight at 3860 kilograms per cubic meter (k/m3). Its cuboid shaped particles have a molar (molecule) mass of 197.841 which easily float to the ground. Arsine gas is much more of a lightweight at 3.186 k/m3. It’s tiny little pyramid particles have a molecular mass of 77.95. About two and a half times heavier than air, it floats around in a blanket near the ground waiting for someone or something that it likes the look of.
Arsenic solid can transform with a bit of heat or acid in to arsine gas. This intruder, completely invisible to the naked eye, apparently smells a bit garlicky or fishy (yuck). Unfortunately, if you can smell garlic/fish at that point, you would have already inhaled enough arsine gas to be completely overwhelmed by it. It doesn’t give you a warning by tickling your nostrils or throat first. According to the CDC ‘Odor is not an adequate indicator of arsine's presence and does not provide reliable warning of hazardous concentrations’.
The Trojan Horse
If arsine gas were a transformer supersoldier it would look for the sneakiest way to get in to the body, so it can use its powers to break in to cells. It slips in through the lungs hiding among oxygen, carbon dioxide and nitrogen molecules. It takes multiple hostages on entry in order to transform itself, and then transforms again in to two arsenic species, then even more species on contact with different targets. It is really all down to which chemicals arsenic finds most attractive. Arsenic has a lot of bedfellows in its rocky origins. In rock formations it can be found with 200+ different elements of metals and minerals. There are some elements arsenic has more affinity with.
How many of these little invaders are problematic? Three molecules of arsine gas per million (ppm) is enough to give us symptoms of ‘headache, thirst, shivering, abdominal pain’, shortness of breath and a general feeling of being unwell. These start any time from 30 minutes after exposure up to 24 hours. To put this in to perspective, there are trillions upon trillions of molecules of air in a cubic centimetre of air AND they only take up 0.1% of the space in that centimetre. All gas molecules are very spread out, so arsine gas molecules will be even more spread out than that, being much fewer in number. We would need to spend a period of time outside in air contaminated with arsine to feel these effects, depending on the amount in the air at the time. Anything that increases respiration rates in an area with ‘thick’ air, such as outdoor sports, would also increase the volume of arsine molecules inhaled.
Arsine gas molecules do not irritate the skin or membranes but can cause a delayed burning effect to the soft lining of the lungs, mouth, nose, trachea and eyes. Contaminated eyes and skin require immediate and thorough washing. Always wash your hands! Soap breaks down the oils on the surface of the skin, which arsine molecules may have temporarily become trapped in. It doesn’t seem like arsine gas can be absorbed by the skin. Liquified arsine gas can cause frostbite.
The amount of arsine in the air, length of exposure and an individual’s ability to detoxify from the gas are all factors in the degree of illness and in the way it manifests. Repeated exposure can also intensify symptoms over a longer period of time. Include in that list of influencing factors delays in symptoms, and people could very well believe they have a cold, flu, stomach bug or other supposed communicable disease. They would expose themselves again and get a little sicker and so on, until the body decides it has had enough and gets properly sick.
Infiltrating the masses
According to Cameo Chemicals arsine gas is the most hemolytic poison known to the chemical industry. What does that mean? It means that this invisible invader does more damage to red blood cells then any other gas molecules used in the chemical industry. Would you believe it is used in the manufacture of semi-conductors? You know those little chips that go in literally everything from televisions to mobile phones. It was considered for use as chemical weapon during the World Wars, but was apparently side lined in favour of other gases such as mustard and phosgene. Lewisite, which used an arsenic chloride compound, was developed during World War I as a blistering agent, but was not used ‘because it hydrolyzes in water and the desired effect cannot be achieved in humid weather conditions’.

When arsine has sunk to just above ground level, these tiny gas molecules will slip past nose hairs and respiratory tract lining, to be absorbed by the gas exchange pores in the lungs. They then have a free pass in to the blood stream. Once arsine molecules have got in to the plasma of the blood stream they start latching on to their first victims. They are extremely attracted to oxygenated red blood cells but not deoxygenated and carbon carrying red blood cells. Somehow the attraction to the oxygen in the red blood cells, gives arsine gas molecules the ability to break in to them. Dracula anyone?
Plundering Oxygen
So what happens when the millions of hemoglobin molecules in a red blood cell, each carrying 4 atoms of oxygen, and hydrogen-heavy arsine gas molecules meet in the cell? The hydrogen bonds to the oxygen, stripping it away from hemoglobin molecules, leaving heme (iron) floating around with no anchor. These fused particles then travel further through the system to create more mayhem.
The end result of this invasion is destroyed red blood cells, a build up of heme in the blood, lack of oxygen in the blood cells, a build up of plateletes desperately trying to fix the leaks, cytokines, white blood cells show up, damaging reactive oxygen species are produced and shrinking of the gaps in vessels. General chaos.
This bloody battle is called hemolysis. Recognised causes are chemicals, noxious gases, some medications, malaria, HIV, H. Influenza, Rocky Mountain Spotted Fever, and some inherited illnesses such as sickle cell anemia and Thalassimia.
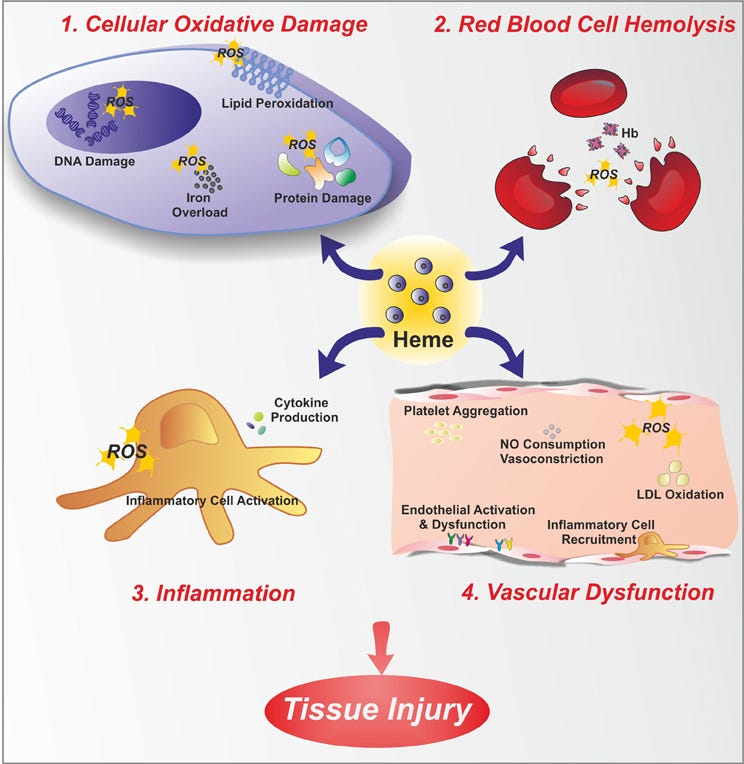
Patients with excessive hemolysis develop hemolytic anemia. They have a large enough reduction in red blood cells that they struggle to get enough oxygen to cells or to remove carbon dioxide. This can result in a broad range of symptoms including weakness, fever, dizziness, confusion, increased heart rate, heart murmur and the released heme can also create inflammation of the liver and spleen.
So whilst the blood is struggling to cope, what is happening to the remaining arsine gas, arsenic species and the bi-product of the fusions? They both might hitch a lift in blood plasma, the arsine gas sneaking in to more red blood and endothelial cells (blood vessel lining), stealing more oxygen, and destroying more cells as they go. Other arsenic species follow, possibly using a slightly different mechanism to get in to the cells at, or may just float along until they reach the liver where they transform in to two new guises.
When hydrogen fuses with oxygen usually water is formed. However, when the body is under attack (oxidative stress), as with arsine gas, hydrogen peroxide also forms. Hydrogen peroxide is a reactive oxygen species (ROS). These bi-products might explain sensations of burning and why fluid accumulates (oedema) in the lungs and tissues. Hydrogen peroxide causes toxin-occupied cells to die preventing them from mutating. It also triggers a cascade of white blood cells, cytokine proteins and bacteria to clean up the mess. This makes the lining of the lungs and tissues inflamed. A high level of gas inhalation can create what is called a cytokine storm. The lungs can swell up so much and effectively ‘flood’. It makes getting air past the cytokine fluid even harder, and therefore further reduces oxygen access to the blood and increases carbon dioxide blood gas, which can cause asphyxiation.
Attacking the engine
Oxygenated blood flows directly from the lungs to the heart through the pulmonary vein in to the left atrium, then the left ventricle of the heart. This blood is carrying with it the arsine gas and recently converted arsenic species. These waste no time in doing damage to the heart muscle, which is called the myocardium. The pericardial function, the sack that contains the heart, seems less immediately affected by the toxins flowing through the heart space. The myocardium responds to the invaders in much the same way as the lining of the lungs and other cells. It becomes inflamed due to high cell death and as a consequence stops working as well as it should. If exposure to arsine gas is high, or any other inhaled toxin for that matter, it can lead to a condition called myocarditis, which can cause heart attack, stroke, heart damage or death.
Leading the charge
The heart then pumps already partially deoxygenated blood together with the arsine and arsenic molecules around the body in to the capillaries, to the brain, muscles, organs, skin, sensory organs, glands and reproductive system. More damage occurs in every area of the body before some toxin is excreted in urine, and the remainder is carried by the blood from the gallbladder, intestines, spleen and pancreas in to the portal vein in to the liver. The liver is the ‘great eliminator’ organ in Chinese medicine.
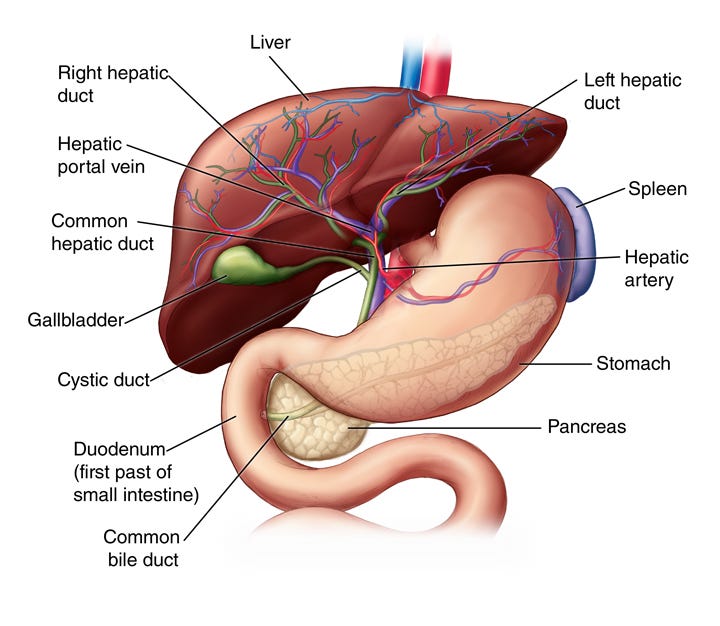
The liver has a number of jobs including processing hemoglobin and bilirubin from red blood cells, removing toxins (poisons), creating immune factors, cholesterol, fat carrying and blood plasma proteins, clotting regulation, Vitamin D3 conversion and storage etc. Toxins and fat reducing compounds are packaged up in the bile to be ejected out of the gallbladder. This bile should break down the fats arriving from the stomach, and the toxins should be bound up by fibre in the food delivery and make it’s way out of the body.
There is a catch. The many incoming (newly converted) arsenic molecules, together with damaged cells from the blood, tissues and free heme also cause lipid peroxides and oxidative stress in the liver. These peroxides are designed to take the heme out of the blood, but are also able to inactivate enzymes and break down proteins and cell walls. In small quantities they can be helpful. Their presence in huge numbers is a reflection of how much damage is being done to cells. This heavy load on the liver can lead to hepatitis (inflammation of the liver) which manifests in fatigue, flu symptoms, dark urine, pale poop, stomach pain, weight loss, yellowing eyes and skin, and generalised itching.
In addition to contending with heme poisoning, liver must convert arsine and bonded arsenic in to two methylated compounds. Methylation of compounds allows them, in an ideal world, to be completely removed from the body through bile and faeces. These compounds are Monomethylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)), which are also, alarmingly, the active compounds used in weed control products. We will talk more about these in the next article.
Once the liver has methylated the remaining arsenic and it has been passed through the gallbladder in to the gut there are a few options. Some will be bound to fibre in the gut and leave by the back entrance. Some will make its way again to the kidneys and some will, depending on diet and the state of the gut, evade capture and recirculate back to the heart to make the whole trip round the body again. This is where things get even more interesting.
The accepted theory is that methylated arsenic allows for effective removal and essentially the job is done. The battle is won and the defeated enemy leaves the battle field. However, more recent research shows that these methylated forms can persist and do more damage in the body.
In the next article we will talk about what MMA and DMA get up to if they make it back in to the blood stream and the chemical interactions that take place between these methylates and different proteins and enzymes to create even more arsenic compounds.
Thanks to Seb for his invaluable contribution as always to this research.
Definitions:
As2H3 - Arsine gas
ppb – parts per billion
ppm – parts per million
ug – micrograms
k/m3 – kilograms per cubic metre
g/mol – Molar (molecular) mass
ROS - Reactive oxygen species




I following fungus (and you too) but looking also on all other possibilities. Reading your posts about arsenic gave me a thought... that we both could be right. So, I entered 'fungus vs arsenic' in a search box and found out something about fungus what I did not know. Some fungi can usefull for removing arsenic and there is also an opposite-some fungi produces arsenic....What if arsenic is produced inside the body? Could be interesting research if you have time for it.
I thinking about this: ''The methylation of As(III) has been observed in a number of organisms, including humans. For example, higher eukaryotes and bacteria have been reported to produce monomethylarsine or dimethylarsine, fungi which produce trimethylarsine (Bentley and Chasteen, 2002; Dombrowski et al., 2005), and methanogens and aerobic eubacteria which produce methylated arsines (Honschopp et al., 1996).'' Volatization of As in less toxic form. So, I understand that fungi could be natures natural defence against Arsenic? More As = more fungi to deal with it? ''Poisoning events due to a gas produced by certain microbes was assumed to be associated with the arsenic in paint. In 1893 the Italian physician Bartolomeo Gosio published his results on "Gosio gas" that was subsequently shown to contain trimethylarsine.[8] Under wet conditions, the mold Microascus brevicaulis produces significant amounts of methyl arsines via methylation[9] of arsenic-containing inorganic pigments, especially Paris green and Scheele's Green, which were once used in indoor wallpapers. Newer studies show that trimethylarsine has a low toxicity and could therefore not account for the death and the severe health problems observed in the 19th century.'' My thoughts is: more As in the body,, more fungi will be there. There is a lot reports of agresive fungal infections during pandemic... ''Microcystis sp. PCC7806, Nostoc sp. PCC7120, and Synechocystis sp. PCC6803 are typical freshwater cyanobacteria. All of them are dominant species in the blue algal eruption. However, little is known about As metabolism in these prokaryotic blue-green algae. In this study, we characterized the patterns and molecular mechanisms of As biotransformation in these three species. By rapidly methylating and volatilizing As, these widespread cyanobacteria may be major contributors to the global As cycle.'' -- So, more As = more cyanobacteria will gather there? ''Cyanobacteria and their toxins can make people sick. In fresh water, such as lakes and ponds, harmful blooms are most commonly caused by cyanobacteria (also called blue-green algae), which are a kind of single-celled organism called phytoplankton. Some cyanobacteria produce toxins (poisons) called cyanotoxins.''