Before we start, a quick reminder of how arsenic interacts with the body. Let’s imagine arsenic is one side of a velcro pad. Most enzymes have disulphide bridges connecting cysteine amino acids together to form structural loops. These are also abundant in mucus forming cells. The cysteines represent the other side of the velcro. The bond that arsenic forms with two cysteine amino acids is very strong. It can stop a protein from functioning, forming new or breaking down proteins, and sometimes making the protein misfold.
Arsenic also knocks out zinc by velcroing itself to 2 cysteines in zinc ‘motifs’. Zinc holds the form of these ‘motifs’ to keep growth control switches in ON or OFF positions, to keep instructions for genes and protein design intact, and many other functions. Arsenate, one form of arsenic, mimicks phosphate molecules, which are used for processes that involve phosphorylation including cell respiration.
In a cancer diagnosis a doctor will take blood, and check for various markers depending on the patient’s symptoms. These tests have to be ordered specifically, they are not part of a normal blood panel. More than one marker is analysed, together with standard blood results, to assess cancer ‘risk’ or potential. The doctor may feel symptoms are related to cancer, if they have ruled everything else out. There are certain signs that doctors are told to watch out for, which might lead them ordering cancer marker tests straight away. This might include an obvious breast mass, a visible skin abnormality, or in the case of the gut, unexplained bleeding.
In this article we will be talking about one of these markers, looking at how it fits in with the arsenic picture and what kind of value it holds as a cancer marker. We also look at how this marker might play a role in the anchoring of cancerous and non cancerous cells, and how those cells got moving in the first place.
Sialyl Lewis A (Carbohydrate Antigen 19.9)
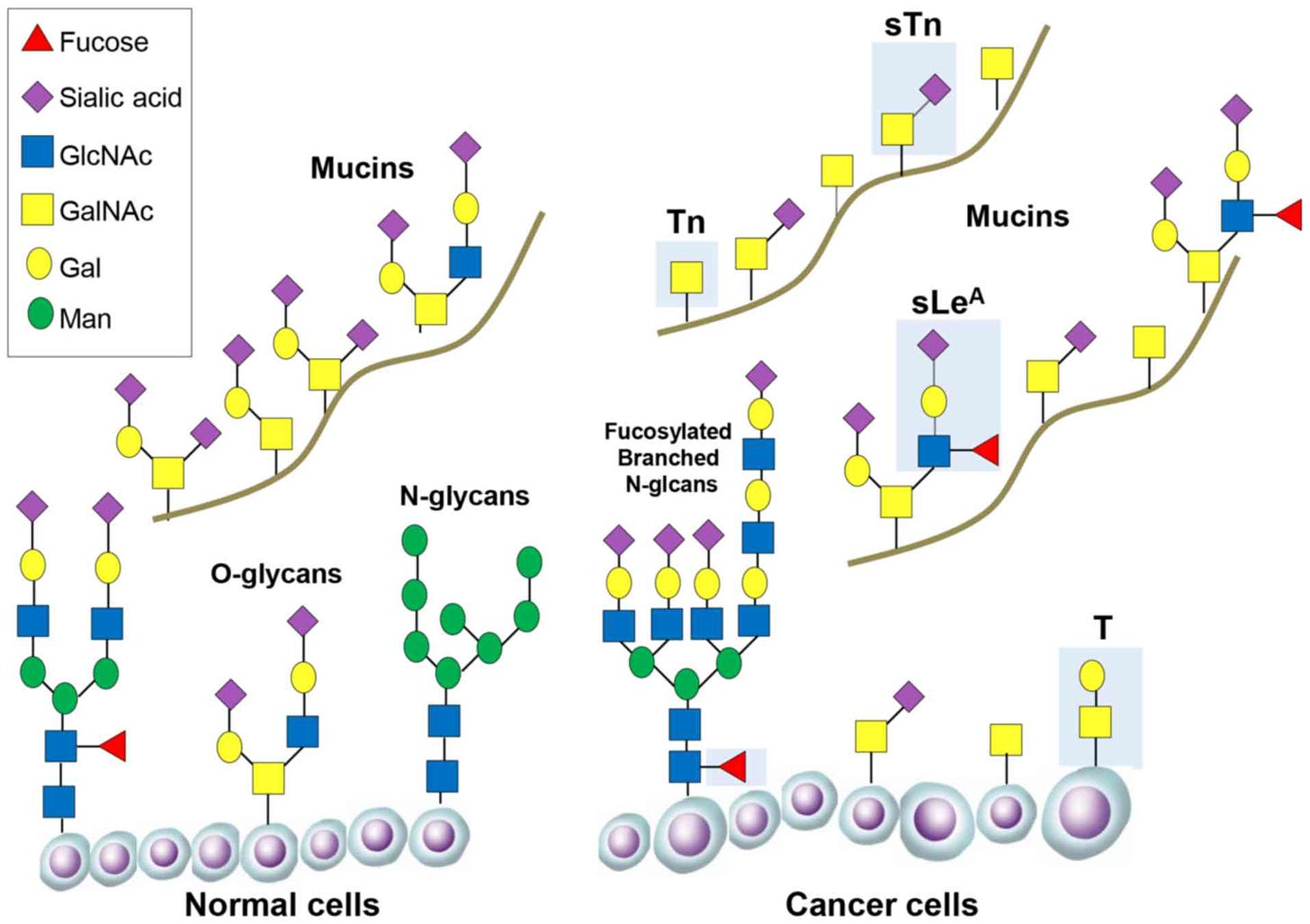
Sialyl Lewis A is a cancer marker for the colon, rectum, intestines, pancreas, bile and pancreatic ducts, oesophagus and stomach. It is also known as Carbohydrate antigen 19.9. An antigen is a molecule that is considered to be toxic to the body, OR it can trigger the immune system to produce antibodies. Sialyl Lewis A is a tetrasaccharide which forms on a chain of sugars called O-glycans, often as part of a mucus membrane forming protein e.g. MUC1. It belongs to a larger group of sialic acids, some of them Lewis based, which often appear together. These acids all have a negative charge so bind to atoms with a positive charge.
In cancer studies this molecule and other sialic acids apparently encourage cancer cell binding. Zhang et al. (2018) found that the level of sialic acids in the blood are associated with increased chance of cancer. Up to 99.5% of sialic acids found in blood are bonded to glycoproteins. Different types of cancer, however, can be associated with high, normal or low levels of these sialic acids.
Sialic acids are not just found in cancer patients though. High sialic acid levels have been found in patients with liver cirrhosis, diabetes, cholitis, pancreatitis, also in Covid-19 patients and a number of other conditions. In the paper ‘Evaluation of serum total sialic acid in moderate COVID-19 patients with and without gastrointestinal tract manifestations’ by Riham Abdel-Hamid Haroun et al. (2021), Covid-19 patients with gut issues had higher levels of serum sialic acid than those without. There seems to be a strong relationship between gut disturbances and these molecules.
In Covid-19 studies sialic acids were involved in the virus sticking to the endothelium. The virus apparently ‘recognises’ sialic acids. If, as we suspect, the virus is simply arsenic-contaminated cells, vesicles and receptors, we would expect the endothelium in the digestive tract to send out a ‘damage’ signal, using cytokines and other markers such as sialyl Lewis A. It is only when arsenic begins to velcro itself to any available cysteine pairs in a haphazard way, that things start to go wrong.
We know that disulphide bridges are critical to the success of ‘viral’ adhesion and entry in to the cell (Grishin et al. 2022). This makes it sound like a deliberate ‘viral’ strategy, when really the endothelium and contaminated cell don’t have much of a choice in the matter where arsenic is concerned.
It is also unclear if the sialyl Lewis A molecule is just a bystander in the whole event, unhappily caught in web of mucusy arsenic bridges, with leukocytes (neutrophils, eosinophils and basophils) unable to get anywhere near the cell surface to fix the damage. Let’s look at that for a moment.
Metal and sialic acid
Whilst we could not find a paper looking directly at arsenic and sialic acid levels in humans (no surprise there), Guner & Bakar (2014) studied effects of cadmium and copper contamination in carp. Cadmium behaves in a very similar way to arsenic. They found that sialic acid increased in blood and brain tissue at the same rate as cadmium and copper increased, ie. they were dose dependent. Importantly, the acids also formed a complex with both cadmium and copper. This means they have the capacity to bind metals. It also appeared that these complexes of sialic acids and metal could be engulfed in to the tissues.
The feature that distinguishes sialyl Lewis A, is the attachment of FUCOSE to the O-glycans sugar of mucus proteins. The proteins that do this is are called N-acetylgalactosamine transferases (Uniprot) and they have five disulphide bridges. You can see how fucose attaches in the diagram below. Fucose is marked in red on what cancer researchers have identified as the cancer-related O-glycan structure.
Fucose is a sugar molecule present in most organisms (Becker & Lowe, 2003). In humans it manages interactions between host tissue and bacteria and, importantly, allows the attachment of leukocytes to cell surfaces. It also assists with tissue regeneration signalling via the NOTCH family of proteins.
Fucoidan, the fucose containing molecule in seaweed and algae, has the potential to remove and contain excess iron and other metals as seen in this paper by Xue et al (2022). ‘Fucoidan is a polysaccharide component of brown algae (such as kelp). It is mainly composed of fucose and sulfate group’. One of the key roles for fucose molecules, attached particularly to sulphur-rich mucin proteins in humans, might be to contain toxic metals, removing them from circulation by encapsulating them. Would this loaded molecule then act as a marker for incoming leukocytes?
Breast cancer cells
Dhar and McAuley (2019) found that MUC1, a mucus protein which sialyl Lewis A forms on, plays a key role in reducing inflammation and infection. This mucin does not feature disulphide bonds, so less of a target for arsenic, which may be why it’s expression reduces inflammation. However, in breast cancer they found the expression of sialyl Lewis A on MUC1 might be a pathway for cancer to grow in the following way. This experiment compares the rolling speed of two different breast cancer cell lines and finds that one type allows the cell to roll slowly enough to adhere to the receptors on the underlying endothelium.
The passing cancer cell attaches to a protein E-Selectin sticking out of the endothelium, that would normally attach to ICAM1 on a leukocyte. Then the E-Selectin ligand on the floating cancer cell also interacts with the sialyl Lewis A on the endothelium. E-selectin has 19 disulphide bridges and ICAM1 has 7.
This video shows how leukocytes should roll along the endothelium and arrive at a gap or a marked spot, which they then stick to and are absorbed through the endothelial wall in to the tissues underneath.
It is unclear whether the cancer cells used in the experiment were screened for the presence of heavy metals or metalloids. It is possible that arsenic contamination plays a role in sticking cancer cells to endothelial receptors. The abnormal shape of the cancer cell without cell death suggests toxic elements may be involved. Red blood cell deformity is show as an effect of arsenic on rat red blood cell shape in this paper by Phangal et al. (2020).
The development of cancer in this case is dependent on there being a free floating cancer cell to adhere to the eipthelium, and not specifically down to the presence of sialyl Lewis A. Saying that, if the cancer cell is cancerous because it has been contaminated with arsenic or similar toxic metal, then the attraction of cell to the sialyl Lewis A is logical.
Random cell movement
Cells do not normally float around freely. Usually they are bound to their neighbour with a network of fibres made from actin and other filament forming proteins. If they do move, like an egg moves from ovary to uterus, this process is controlled by a protein called n-cofilin. N-cofilin breaks down actin fibres to allow the cell to be moved. When n-cofilin has finished its job it is phosphorylated, and broken down so it can no longer dissolve fibres holding cells in place. Arsenic can mimick phosphate, preventing phosphorylation and other phosphate dependent processes.
If N-cofilin cannot be inactivated because arsenate is high and phosphate is in short supply, levels will remain high, increasing the number of released cells. The role of N-cofilin in cancer and metastisis is discussed more in the article ‘Cofilin: A Promising Protein Implicated in Cancer Metastasis and Apoptosis’ by Xu et al. (2021).
In high arsenic concentrations, while sialyl Lewis A is holding out its fucose red flag waiting for a leukocyte to arrive, n-cofilin has been beavering away dislodging cells. The increased blood, plasma, and cytokine flow to the site of tissue damage might also carry with it a sudden influx of random floating cells. The random cells have been released by rogue out-of-control N-cofilin because the phosphate to keep it in check has been kicked out by arsenate.
By the time these cells arrive at the red flag, they have arsenic velcro pads stuck all over them. Inflammed tissues reduce the gap through which the cells have to squeeze, pushing them closer to the wall where more sticky cysteine receptors are present, some with and some without arsenic already attached. So it may not just be irregular cancerous cells that stick, but normal shaped cells that have been let loose from their moorings by N-cofilin. We will talk about what defines cancerous cells in the next article.
Many factors that promote the filament forming actin are significantly reduced in some cancers. As ATP binding (phosphate-rich molecule) is required to regulate the formation of actin fibres, where phosphate levels are low because of arsenate, actin activity would also be compromised.
Phosphate in Cancer
To cross check how phosphate loss contributes to cancer we checked for trends in phosphate levels in cancer patients, bone density loss and increase of fractures. Phosphates are essential for healthy bone formation and maintenance as seen in this paper by Hughes et al (2019)
Low phosphate levels are common in cancer patients according to Adhikari et al (2021) The condition is blamed on a number of factors, none of them looking at the role of arsenate. ‘Hypophosphatemia, defined as serum phosphorus ˂2.5 mg/dL, is a common occurrence in cancer patients and is associated with increased morbidity and mortality. Phosphorus is essential for the normal physiologic function of all cells and its homeostasis is frequently interrupted by cancer and cancer therapy.’
They also find that low phosphate leads to a condition called osteomalacia, which means a softening of the bones. This condition can also lead to rickets. In cancer patients Adhikari et al (2021) find that ‘patients present with bone pain, gait disturbances, pathological fractures, height loss and proximal muscle weakness’ and ‘Chronic hypophosphatemia leads to osteomalacia that appears as osteopenia and pseudofractures on radiographs’.
Therefore it is likely that arsenate-induced loss of phosphate leads to reduction in bone density, softening of the bone and fracture, AND prevents breakdown n-cofilin encouraging high levels of abnormal cell movement.
Transplant rejection
In a study by Turunen et al at Osaka University Medical School (1995), researchers looked at cardiac transplant rejection. The transplanted tissue is shown to be overrun with lymphocytes, these are the T, B and natural killer cells that are first responders to a site where foreign protein or toxins have been detected. At the same time sialyl Lewis A and X levels were found to be high on the donor heart tissue during rejection of transplant.
The sialyl Lewis molecules and high levels of leukocytes caused the host tissues to begin rejecting the transplant. Only anti-lewis and anti leukocyte receptor treatment could stop the process of rejection.
Pregnancy and Miscarriage
MUC1 plays a role in the endometrial epithelium in pregnancy, and is present in abundance at implantation stage. MUC1 naturally generates sialyl Lewis A and X in the endometrium specifically at implantation stage of the menstrual cycle. At this stage there is a degree of inflammation at the site of implantation which is required to encourage proper implantation.
In cases of repeat or spontaneous (single) miscarriage from 6-13 weeks, as seen in this study by Ma et al. (2021) there was less cell adhesion than needed to support the pregnancy. The production of Sialyl Lewis A, X and Y were all reduced and X and Y could not be seen in the placental villi of both miscarriage groups. The assumption is that the lack of sialyl Lewises prevented placental adhesion leading to miscarriage, but if we compare this study with the study on heart transplant rejection that makes little sense.
Instead, could sialyl Lewises actually be designed to protect the placenta from toxins or bacteria coming from the uterus and mother’s blood supply? Without these molecules the placenta could be laid open to infection and contamination. In the study placental tissue was markedly inflammed and the villi depth was reduced, in the same way that villi depth is reduced in the gut by errosion by arsenic and other toxic metals.
Interestingly, in an experiment targeting and blocking sialyl Lewis A with an anti Lewis agent GM35, neutrophil migration in to underlying human tissue was also blocked.
The protein NEU1 was also increased in spontaneous miscarriage. NEU1 (oxytocin-neurophysin) binds oxytocin at the N-glycan, but contains 8 disulphide bridges, therefore becomes a target for arsenic. Sometimes in high arsenic concentrations we see an increase in specific protein production in an attempt to compensate for losses to arsenic attachment. Arsenic can also cause increased inflammation of the placenta as detailed in this paper by Ahmed et al. (2011)
Protection from bacteria
Sialyl Lewis A and X have also been shown to be critical to preventing invasion of epithelial cells in the mouth by the bacteria T. Forsythia. There is evidence some bacteria are capable of breaking sialic acids away from their proteins. Researchers Frey et al. (2018) analysed ‘the role of Lewis antigens (SLeA and SLeX) both as catalytic substrates and ligands for the NanH-CBM, and also as key ligands for host-pathogen interactions during cellular invasion.’ Sialyl Lewis A reduced gum invasion by bacteria dramatically, and both A and X showed they were more resistant to bacterial breakdown than the other sialic acids. This is further evidence that sialyl Lewises play a strong role in tissue defence.
Leukocyte failure
Sialyl Lewis A seems to recruit leukoctyes to damaged tissue, but it doesn’t explain why leukocytes fail to arrive at the scene, allowing arsenic to stick cancerous and non cancerous cells to the site instead. ‘Leukocyte adhesion deficiency’ by Vaillant and Ahmad (2023) looks at what prevents leukocytes from sticking to damaged endothelia in this condition. The leukocytes may not be able to migrate to the site of injury, for example if leukocytes are not getting the T-Cell signals required (see the vaccines and viruse series for more).
There might also be an error in the gene coding for beta integrin adhesion molecules particularly beta integrin 2, or they are compromised in some way. Beta integrin 2 enables leukocytes to stick to their target AND helps them move to the site of cellular damage. When we look at the structure of beta integrin 2 we can immediately see why these proteins might be disabled an arsenic heavy environment. This molecule has no fewer than 28 disulphide bridges. As soon as it is exposed to high arsenic concentrations, they become saturated. Leukocytes would have no clean beta integrin 2 to help them stick to receptors.
In summary, sialyl Lewis A seems to be a marker for protein and cell damage, and for the presence of foreign pathogens and antigens, assisting rather than hampering the immune system. The adhesion of wandering cells to sialyl Lewis A, may simply be down to the stickiness of a positive arsenic ion to the negative charge presented by the molecule. The attachment to and incorporation of arsenic may well be a deliberate part of immune defense. The wandering cell attached to that arsenic might be coincidental, but at the same time removes it out of circulation. Where cancerous cells and metastisis are concerned, this could be a good thing. The issue comes when the level of wandering cells increases, leading to the formation of a tumour, particularly in areas of high inflammation.
Raised levels of sialyl Lewis A could well be a strong indicator for the presence of arsenic or other heavy metals in the body. Other markers such as low phosphate, low leukoctye count, low beta integrin and high n-cofilin would also support this diagnosis. These markers together would not necessarily indicate the presence of cancerous cells but could act as a red flag for toxicity with cancer-causing potential.