We have heard about how lungs are affected by both ionising radiation and Covid-19. But what happens when the blood is exposed to either of these 'pathogens'? We take a look in this article at the white blood cells, red blood cells, their components and how changes in these can directly affect the heart.
Table 3-3 on page 99 in this paper from the CDC gives a summary of the generalised effects and timeline of radiation poisoning dependent on dosage. We can see from the chart that a number of main effects on the blood (hematopoietic) system occur, including leukopenia, purpura and hemorrhage.

White Blood Cells
Leukopenia, a reduction in white blood cells, occurs in people exposed to radiation and chemical poisoning, alongside other causes including reactions to certain drugs, autoimmune conditions and stem cell transplantation. White blood cells (leukocytes) are produced in bone marrow along with red blood cells. Children, significantly, have a greater mass of blood cell producing bone marrow in relation to their body size. As we grow older the composition of our bone marrow changes and we loose red and white blood cell producing marrow, which is replaced by fats instead.
The different categories and roles of cells are detailed below. Lymphocytes are made up of B cells, which produce antibodies to attack invading pathogens and T cells which destroy human cells that have been taken over by invading pathogens.

According to Radiation Emergency Medical Management, upon exposure to H2 and H3 (H1 being low and H4 high) levels of radiation, lymphocytes drop and remain low, according to the dosage received. Granulocytes generally show an initial rise followed by a significant drop, which begins to recover around 30 days after exposure. Lower levels of lymphocytes and granulocytes increase the chance that the body will develop bacterial, fungal or parasitic infections. These infections are opportunistic, but bacteria are also signaled to deal with dead cellular matter, which indicates that cell death would be significant where infection sets in.

In the paper ‘Hematological manifestations of COVID-19’, lymphopenia and leukopenia (low levels of lymphocytes and white blood cells in general) were identified as common in Covid 19 patients. In another paper from Brookdale University Hospital, low lymphocytes showed in 96% of severe Covid-19 cases and 80% of milder cases. CD8+ T, or Killer T cells, deteriorated due to continued inflammatory signals leading to exhaustion. This condition is also found in radiation exposure, and in extreme cases full recovery of these killer T cells will not occur before 180 days post exposure.
Platelets
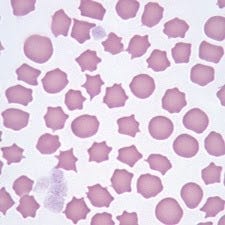
Platelets, which are in charge of forming blood clots (thrombosis) at injury sites, increase slightly between days 1-10 of radiation exposure, then drop considerably, only starting to recover from day 30. Reduced platelet levels in the blood is called thrombocytopenia. We would likely see clotting in the first 10 days after exposure, followed by increased chances of bleeding, which might show in the form of bruising, internal hemorrhage and purpura. Purpura are burst blood vessels or capillaries under the skin, in organs or mucus membranes. These can show as tiny pin pricks in size all the way up to large pools of blood.
In Covid 19 patients, in mild cases we would see a slight rise in clot forming platelets, which would then go back to normal. In more severe cases, after an initial rise, again increasing the chances of clots forming, we would see a condition develop called thrombocytopenia, or the destruction of platelets and a reduction in platelet numbers. See Image 4 below. This would lead to hemorrhage, or bleeding, increasing with severity of Covid infection. In Covid-19 patients it is recognised as leading to purpura which manifests in the form of ‘petechiae or purpuric rashes’; pin-prick bleeding under the skin or larger pools of bleeding under the skin.
A very similar pattern is seen in patients with H1 level radiation poisoning, an initial climb, then normalisation. In H2 and H3 we would see an climb to around days 10-16, then a drop to well below normal levels, which then normalise between 30-40 days. The timescales shown in this chart of Covid-19 patient platelet analysis shows an increase from day 1-7 after admission (not upon exposure), with a gradual reduction between day 8-18, a dip at day 19 and return to normal by day 20. Purpura, as per image 1, is also often present in more severe exposures.

In this paper about Belgian Covid-19 patients, hospitalisation occurred between 3 and 10.4 days from onset of symptoms. If we take an average admission date at day 7 of onset of symptoms (not exposure, which may have happened a number of days before), then the time to recovery of platelet levels potentially matches that of patients suffering from radiation exposure.
Red Blood Cells
Finally we need to look at changes to red blood cells, or erythrocytes. These cells are responsible for carrying oxygen to cells from the heart/lung system and carbon dioxide back to the hearth/lung system, for disposal through exhalation. These are also produced in the bone marrow. Red blood cells last for approximately 120 days, before they are removed from the body in the spleen, liver or bone marrow. This is a great video on explaining how red blood cells are produced and recycled in the body.
Exposure to toxins and radiation can cause crenation (or burr type spikes) on red blood cells. Crenation occurs when a blood cell is near the end of its life, in this case prematurely. Mildly affected cells can recover, extremely deformed cells cannot. This paper by the Iranian Journal of Medical Physics goes in to more detail of the effects of X-ray radiation on red blood cells. Numbers of crenated blood cells increase when exposed to Reactive Oxygen Species (ROS), which are made when human cells are exposed to radiation. This leads to increased red blood cell death, reducing the blood’s oxygen and carbon dioxide carrying capacity.
Radiation also directly weakens the blood cell membrane making it more permeable. Blood is irradiated using gamma rays to sterilise blood components before transfusion in to patients. In the paper ‘The Effect of Pre-Storage Irradiation Blood on Quality of Red Blood Cells’ irradiated blood was found over time, to have increased potassium, free hemoglobin and dead red blood cells in the plasma (the liquid in which all blood cells are suspended) and a reduced amount of sodium. This indicates that the cells were leaking potassium and retaining/absorbing sodium, which led to significant cell death in comparison to the experiment control.
In Covid-19 we know that oxygen levels reduced in patients according to severity of infection, making them hypoxic. We see in this article from CU Anschutz Medical Campus that researchers of Covid-19 realised the condition was causing this by weakening the cell membranes of red blood cells.
Hypokalemia (low potassium in the blood) in this study is present in a considerable number of Covid-19 patients. 24.3% of Covid patients suffered Hypokalemia and only 4.15% suffered from hyperkalemia (high potassium in the blood). There are limited ways in which hypokalemia could happen: intensive prolonged exercise, severe fluid loss due to vomiting and diarrhoea, kidney disorders, intestinal obstruction or infections. We have seen the kidneys affected in the course of illness progression, but it is possible that this is a consequence of extreme system detoxification, rather than existing kidney defects.
Alternatively another study sites a potential cause being ‘An intracellular shift of the potassium can also lead to severe hypokalemia.’ Is it possible that the hemolysis (red blood cell death) seen in Covid-19 patients is happening because of leaky red blood cell membranes in the same way that occurs to blood when irradiated?
Also in Covid-19 patients, we are seeing very immature red blood cells in high levels (60%), flooding in to the blood stream. Researchers from the University of Alberta in Canada noted this was very unusual, indicating that bone marrow was being affected by the illness. What if the mature red blood cells in the blood system were being destroyed in such great numbers that the body had no option but to release immature cells? Immature blood cells are not able to carry oxygen however, so the issue would still not resolve, and the patient could remain hypoxic.
So as we can see, severity of disease, or exposure, whether from Covid-19 or radiation, affects all aspects of blood composition and the production of these cells in the bone marrow.
The Heart
How do these changes in the blood affect the heart? Potassium and sodium balance is crucial to maintaining the structure of cell membranes, (we have seen what happens to the shape of cells when this balance is impaired), it regulates blood pressure and provides electrical energy for muscular, neural and other tissues to function. The Sodium/Potassium pump is responsible for keeping this balance in check. If the cell is damaged, particularly in the membrane where the sodium/potassium pumps are located, the pumps fail and leakage occurs. As potassium maintains more ions in the cell than sodium, this causes the cell to loose potassium.
As you can see in this video, potassium is crucial for the Sinoatrial Node (pacemaker) in the heart to function. It controls the build up of electrolyte based charges in the node cells, that cause the heart to have a contraction or beat. This then moves the blood. When there is an lack of potassium in this node, atrial fibrillation occurs. Atrial fibrillation, or a rapid irregular heart beat, is the node’s attempt at compensating for lack of energy required to fire regular heartbeats. If the node is lacking in potassium (or indeed in sodium) for a prolonged period of time this would put stress on the heart muscle. The irregular mode of pumping could also cause blood clots to form. In Covid-19 and irradiation we know already that platelets rise in the first 10 or so days of infection. If potassium is being lost simultaneously due to cell membrane damage, this further increases the potential for blood clots.
As we have seen in one of our previous articles, inflammation is caused by the gathering of white blood cells, specifically neutrophils, to the site of damage. If damage is extensive, the inflammation will also be. Inflammation in clinical descriptions usually end in the letters ‘…itis’. So tonsilitis, inflammation of the tonsils, bronchitis, inflammation of the bronchus, arteritis, inflammation of the arteries and so on. In clinical research of Covid-19 patients, inflammation is seen to affect not only the respiratory tract but other systems and organs as well. Some patients develop a condition called Multisystem Inflammatory Syndrome (MIS), whilst some show inflammation in one or two specific areas such as the eyes, gut or heart.
We have seen above that a rise in platelets and blood clotting, is an early manifestation of Covid-19 and radiation exposure. We also know that purpura, hemorrhage and micro-bleeds are a later feature of exposure in both. The Global COVID-19 Thrombosis Collaborative Group found that thrombosis (clotting) was a significant risk factor in Covid-19 in their article from September 2020. They also list cardiogenic shock, myocardial injury, myocarditis (inflammation) and myocardial infarction (tissue death due to hypoxia) as effects.
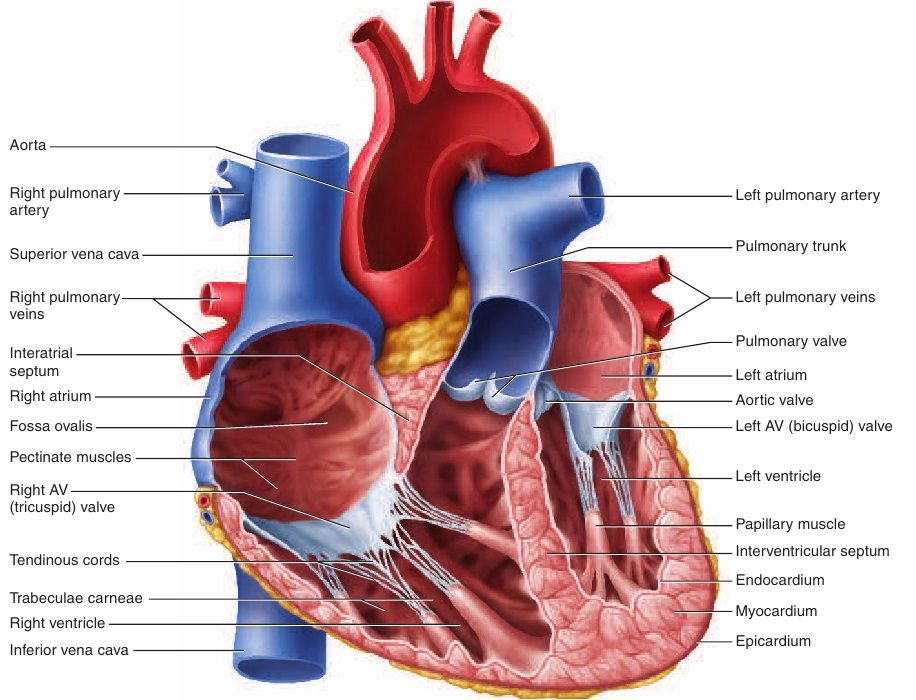
The myocardium is the blood-vessel rich muscle in the heart, responsible for contracting blood through the various chambers of the heart to the lungs to pick up oxygen and dump carbon dioxide, then through the arteries to the cells of the body to do the reverse. In order to function, this cardiac muscle needs a constant supply of oxygen from red blood cells. This video explains how the heart muscle is supplied with blood.
As we have seen above, when red blood cells are exposed to radiation they leak potassium and begin to crenate and die. The same damage might also be occurring to other cells including muscle cells in the heart should exposure be high enough. As shown above in the section on red blood cells it seems a similar thing may also be happening to Covid-19 patients.
Fewer red blood cells, means less available oxygen in circulation, leading to potential cell death in the heart muscle (and elsewhere), and the need for the heart to work much harder to compensate. Not only that, but the micro clotting/micro hemorrhages in blood vessels cause a cascade of white blood cells responding to fix the damage. This in turn causes inflammation, which is known as myocarditis.
Numerous cardiac conditions are associated with ionising radiation exposure as we can see in ‘Radiation-Induced Heart Disease: A Clinical Update’. Functional areas affected include ‘pericardium, myocardium, valves, and coronary vessels with pericardium’. Illnesses found in the acute stage of exposure show pericarditis and cardiomyopathy (disease of the heart muscle or myocardium). In this study on Covid-19 patients, again by the Jama Network, they found that 78% patients exhibited heart inflammation in the form of pericarditis and myocarditis.
Summing up
Radiation induced conditions related to the cardiovascular system and blood are as prolific as those found in Covid-19. We have found a number of common factors in this article, though are sure there may be elements of difference. The research done for this article is necessarily relatively shallow in depth.
Where research papers about Covid-19 refer to ‘Virus’ and ‘Viral receptors’ causing cell damage, cell death, hemorrhage and clotting, papers on radiation talk about direct and indirect effects of radiation causing cellular damage, cell death, hemorrhage and clotting. The difference is simply defined on one side by the ‘virus’ particles, and on the other by the ‘exosome’ particles and radiation waves.
As always, thank you to all who join in the bouncing of ideas around, and also make sure everything is readable!
Coming soon -
Part 3 of the Radiation/Covid Comparison on the human body looking at the neurological impacts, and a separate look at the art of silent war.




Excellent as ever Caroline. It certainly makes a lot more sense than the official narrative
https://pubmed.ncbi.nlm.nih.gov/34984951/